We have seen recently how easily false narratives get picked up and repeated so many times that eventually it is believed as true. Perhaps this is an idea that was borrowed from cooking. Cooking has long been rife with traditions and ideas that have been passed down from generation to generation that while the original reason for doing something a certain way may have been valid at the time, the modern day reasoning has morphed into something completely different. I am going to test a few of these cooking claims that seem odd to me and see if they are in fact true.
A number of years ago I heard Bobby Flay on an episode of Boy Meets Grill claim that cold water boils faster than hot. He did add the caveat that it was something a culinary school instructor told him and that he didn’t know if it was true. I have seen a few articles and videos (including on Food Network) that have debunked this myth, but have not provided much in the way of data to support their claim. More recently I was watching an Americas Test Kitchen where they claimed that you should use less water when boiling your food. The reason for this being is that it takes a larger volume of water to return to a boil than a smaller volume of water. These seem to be easily provable/disprovable claims, so I am going to pour myself into this and boil water, lots of water. As an added bonus, this blog will also prove that a watched pot does indeed boil.

For this analysis I performed a total of 24 tests detailed in the chart. This had a cumulative active boiling time of over 3 hours. Yes, I do have a life — just not on this particular day. All tests were ran using a 7 cup sauce pan and a digital instant read thermometer. I took time measurements at each 10 °C rise in temperature, up to 98 °C. I only measured up to 98 °C versus 100 °C for a couple of reasons. First, because I am not at sea level, I will never reach 100 °C. Second, my true boiling point is just slightly higher than 98 °C, but I could not consistently achieve the temperature I measured for boiling with all volumes of water. For starting temperatures I chose 20 °C and 40 °C, as those 2 temperatures are pretty close to the coldest temperature and the hottest temperature coming out of my tap and they make it easy to do 10 °C increments. All of my data was then uploaded to Tableau Public, which is free for anyone to use, to create the visuals in this blog.

Okay enough with the boring stuff — what were the results? Well, as I mentioned up top, warm water does indeed boil faster than cold water. Not only does cold water not boil faster than warm water, as the volume increases, the time to reach a boil between cold and warm water also increases. Perhaps there was, or possibly still are good reasons to to use cold water versus warm water, but it is most certainly not because cold water boiled faster.
[iframe src=”https://public.tableau.com/views/WaterBoiling/AverageTimetoBoil?:showVizHome=no&:embed=true” width=”100%” height=”650″]
As you can see in the visual below, there was some variability in the times it took to get to each checkpoint temperature, particularly when starting from 20 °C. The one thing that is consistent though are the overall slopes of each line. I suspect much of the variability in the times to reach each checkpoint temperature are do to slight inconsistencies with pan temp and gas setting on the heat source, since the times for 40 °C starting temperature are pretty consistent.
[iframe src=”https://public.tableau.com/views/WaterBoiling/AverageTimeToBoil2?:showVizHome=no&:embed=true” width=”100%” height=”600″]
An interesting thing to note is that temperature rise is not completely linear. The first 10 °C of temperature rise takes longer compared to every other temperature checkpoint up to 80 °C. This is consistent across the range of temperatures and volumes. It is probably due, at least in part, to the fact that it takes a bit of time to transfer heat through the pot material and into the water. However, the last 18 °C, from 80 °C — 98 °C also take longer to achieve. It could be due to the heat lost to the environment has a greater effect at higher temperatures, or the water getting closer in temperature to the temperature of the heat source. However, as these are only observations, I am not sure exactly what causes this.
I also find it interesting, yet not totally unsurprising, that when I increased the volume of water, I got roughly proportional increases in the amount of time it takes to boil. The basic formula to estimate the time to boil would be to take the percent increase in volume and divide by 2. Since 4 cups is a 100% increase in volume over 2 cups, (100% / 2 = 50%) more time for 4 cups to reach a boil. When the volume is increased from 4 cups to 6 cups, that is a volume increase of 50%, so it takes about (50% / 2 = 25%) more time to boil.
So a good rule of thumb, if you know the approximate time to boil for one volume of water would be to apply the following formula*.
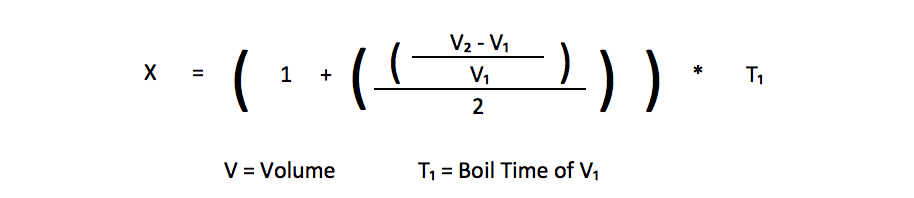
Based on that calculation I plotted out what the calculated time to boil is for 3,4,5 and 6 cups. I then conducted a few tests at the 3 and 5 cup volumes to test how well the formula works. As it turns out, it works pretty well, particularly when the starting temperature of the water is 40 °C. There is a slight difference between the calculated time to boil and the actual at 6 cups with a 20 °C starting temperature. As you will see later in the post, I had a pretty substantial standard deviation in time to boil with that volume and starting temperature. I suspect if I had repeated that test more times I would have come closer to the calculated time. One quick disclaimer is that while this formula is true for the pot I was using, it may be different for a different size pot with different sized bottom. If anyone wants to test this themself and let me know how it worked, I would be interested in hearing about it.
[iframe src=”https://public.tableau.com/views/WaterBoiling/TTBvsEst_TTB?:showVizHome=no&:embed=true” width=”100%” height=”650″]
Since my testing method wasn’t as consistent as it possibly could have been, I will include a chart of the standard deviation of my times for all of the experiments I ran. For tests where the starting temperature was 40 °C it looks like I got pretty consistent results. Depending on the volume of water I recorded a range of about 5 — 12 seconds between the shortest time to 98 °C and the longest. Things get decidedly less consistent when starting from 20 °C, with a range of about 12–44 seconds at 98 °C. These inconsistencies in times to boil likely have some effect on the percentage increases reported from one volume to the next, particularly for the 6 cup amount with starting temperature of 20 °C.
Lastly, let’s test whether Americas Test Kitchen’s claim is true. To simulate dropping food into water to boil, I used ice cubes. I performed this test with 2 ice cubes equalling 1/4 cup of liquid and 4 ice cubes equaling 1/2 cup. So did it take longer for larger volumes of water to return to a boil? No, it did not. In every case, the greater the volume, the faster it returned to a boil. As you can see from the chart, the minimum temperature I recorded for each test was greater with each increase in volume. So the less heat the larger volumes lost more than offset the time it takes for larger volumes of water to boil. Did ATK just make that claim because, as I have shown above, it takes longer to boil larger quantities of water and didn’t actually test how it works in practice? I don’t know, but as I have shown here with my process is that it isn’t true. Again, I welcome anyone else’s results should they want to try this out for themselves.
[iframe src=”https://public.tableau.com/views/WaterBoiling/TimeToReturnToBoil?:showVizHome=no&:embed=true” width=”100%” height=”650″]
Check out our collection of tip posts here. Subscribe to have them delivered to your inbox here.
Okay, so now that you are drowning in data, I think it is time to wrap this up. As I have proved here, a watched pot does indeed boil, it boils faster if you start with warm water and it returns to a boil faster with more water in the pot. So why do false claims like these get so much traction and imbed themselves in our fact bank? I don’t know, but at least if you have the time and motivation, they can be proved right or wrong. This may be more about boiling of water than you ever wanted to know, but at least you can’t accuse me of having dry content.
Check out our collection of tip posts here. Subscribe to have them delivered to your inbox here.